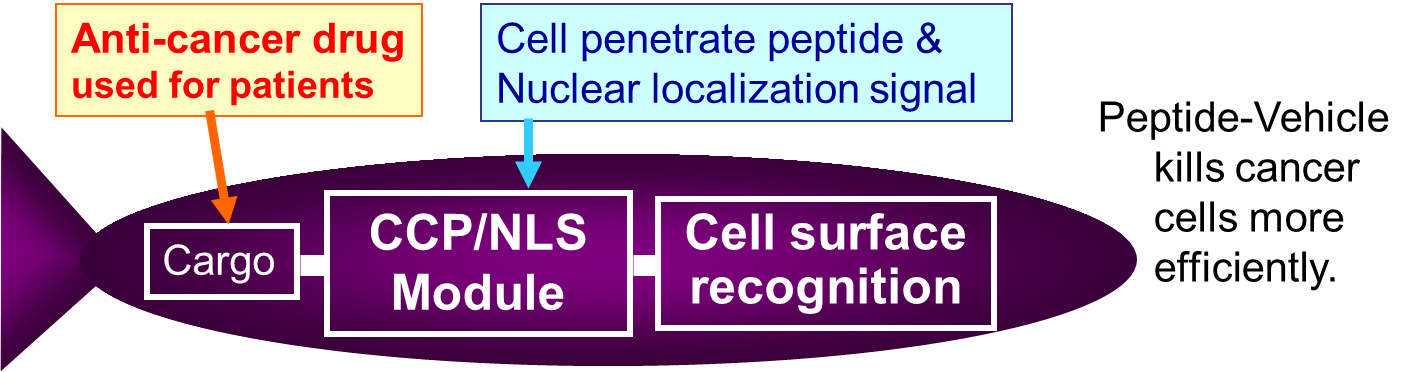
Overview
In the three years from FY 2008 to FY 2010, we promoted Okinawa Innovation Research Consignment Project “Developing cancer therapeutic drugs and test methods using peptide libraries”. We designed a peptide-vehicle in which a cell surface recognition peptide, a cell penetrating peptide and an anticancer agent are conjugated as a candidate substance for “cell targeting therapeutic drug”, and studied to construct a library. We will provide “Peptide-Vehicle Library” constructed based on the results. In recent years, problems such as QOL decrease due to side effects, patent disappearance of anticancer substances are a problem, and it is expected that drugs will be delivered that anticancer drugs specifically to cancer cells. We hope to use it as a tool of drug discovery research.
Reference Hirata, A., Nokihara, K. Construction of peptide-vehicles, bioconjugates having modules of cancer cell surface capture and cell-penetrating peptide with anticancer agents, Tetrahedron Lett. 2014, 55, 4091-4094.
| Peptide No. | Sequence(C terminal amide) |
| HiPeV-001F | αTAMRA-GPKSKRKVYGRKKRRQRRR |
| HiPeV-002F | αTAMRA-GPKSKRKVRQIKIYFQNRRMKWKK |
| HiPeV-003F | αTAMRA-GPKSKRKVRRRRRRRRR |
| HiPeV-004F | αTAMRA-GPKSKRKVRRRRNRTRRNRRRVR |
| HiPeV-005F | αTAMRA-GKKNQKKRYGRKKRRQRRR |
| HiPeV-006F | αTAMRA-GKKNQKKRRQIKIYFQNRRMKWKK |
| HiPeV-007F | αTAMRA-GKKNQKKRRRRRRRRRR |
| HiPeV-008F | αTAMRA-GKKNQKKRRRRRNRTRRNRRRVR |
| HiPeV-009F | αTAMRA-GPKSKRKV |
| HiPeV-010F | αTAMRA-GKKNQKKR |
| HiPeV-011F | αTAMRA-GYGRKKRRQRRR |
| HiPeV-012F | αTAMRA-GRQIKIYFQNRRMKWKK |
| HiPeV-013F | αTAMRA-GRRRRRRRRR |
| HiPeV-014F | αTAMRA-GRRRRNRTRRNRRRVR |
| HiPeV-015F | TAMRA-G-YKQC(Acm)HKKGGKKGSG |
| HiPeV-016F | TAMRA-cNGRGEQcYGRKKRRQRRR |
| HiPeV-017F | TAMRA-cNGRGEQcRQIKIYFQNRRMKWKK |
| HiPeV-018F | TAMRA-cNGRGEQcRRRRRRRRR |
| HiPeV-019F | TAMRA-cNGRGEQcRRRRNRTRRNRRRVR |
| HiPeV-020F | TAMRA-YGRKKRRQRRRcNGRGEQc |
| HiPeV-021F | TAMRA-RQIKIYFQNRRMKWKKcNGRGEQc |
| HiPeV-022F | TAMRA-RRRRRRRRRcNGRGEQc |
| HiPeV-023F | TAMRA-RRRRNRTRRNRRRVRcNGRGEQc |
| HiPeV-024F | FAM-GYGRKKRRQRRR |
| HiPeV-025F | FAM-GRQIKIYFQNRRMKWKK |
| HiPeV-026F | FAM-GRRRRRRRRR |
| HiPeV-001D | C(S-EMCA-Gly-CPT)-YGRKKRRQRRR |
| HiPeV-002D | C(S-EMCA-Gly-Iri)-YGRKKRRQRRR |
| HiPeV-003D | C(S-EMCA-Gly-Eto)-YGRKKRRQRRR |
| HiPeV-004D | C(S-EMCA-Gly-Pac)-YGRKKRRQRRR |
| Peptide No. | Sequence(C terminal amide) |
| HiPeV-005D | Cys(S-EMCA-Gly-CPT)-YGRKKRRQRRRcNGRGEQc |
| HiPeV-006D | Cys(S-EMCA-Gly-Iri)-YGRKKRRQRRRcNGRGEQc |
| HiPeV-007D | Cys(S-EMCA-Gly-Eto)-YGRKKRRQRRRcNGRGEQc |
| HiPeV-008D | Cys(S-EMCA-Gly-Pac)-YGRKKRRQRRRcNGRGEQc |
| HiPeV-009D | Cys(S-EMCA-Gly-CPT)-RQIKIYFQNRRMKWKKcNGRGEQc |
| HiPeV-010D | Cys(S-EMCA-Gly-Iri)-RQIKIYFQNRRMKWKKcNGRGEQc |
| HiPeV-011D | Cys(S-EMCA-Gly-Eto)-RQIKIYFQNRRMKWKKcNGRGEQc |
| HiPeV-012D | Cys(S-EMCA-Gly-Pac)-RQIKIYFQNRRMKWKKcNGRGEQc |
| HiPeV-013D | Cys(S-EMCA-Gly-CPT)-RRRRRRRRRcNGRGEQc |
| HiPeV-014D | Cys(S-EMCA-Gly-Iri)-RRRRRRRRR cNGRGEQc |
| HiPeV-015D | Cys(S-EMCA-Gly-Eto)-RRRRRRRRRcNGRGEQc |
| HiPeV-016D | Cys(S-EMCA-Gly-Pac)-RRRRRRRRRcNGRGEQc |
| HiPeV-017D | Cys(S-EMCA-Gly-CPT)-RRRRNRTRRNRRRVRcNGRGEQc |
| HiPeV-018D | Cys(S-EMCA-Gly-Iri)-RRRRNRTRRNRRRVRcNGRGEQc |
| HiPeV-019D | Cys(S-EMCA-Gly-Eto)-RRRRNRTRRNRRRVRcNGRGEQc |
| HiPeV-020D | Cys(S-EMCA-Gly-Pac)-RRRRNRTRRNRRRVRcNGRGEQc |